REPORT OUTLOOK
| Market Size | CAGR | Dominating Region |
|---|---|---|
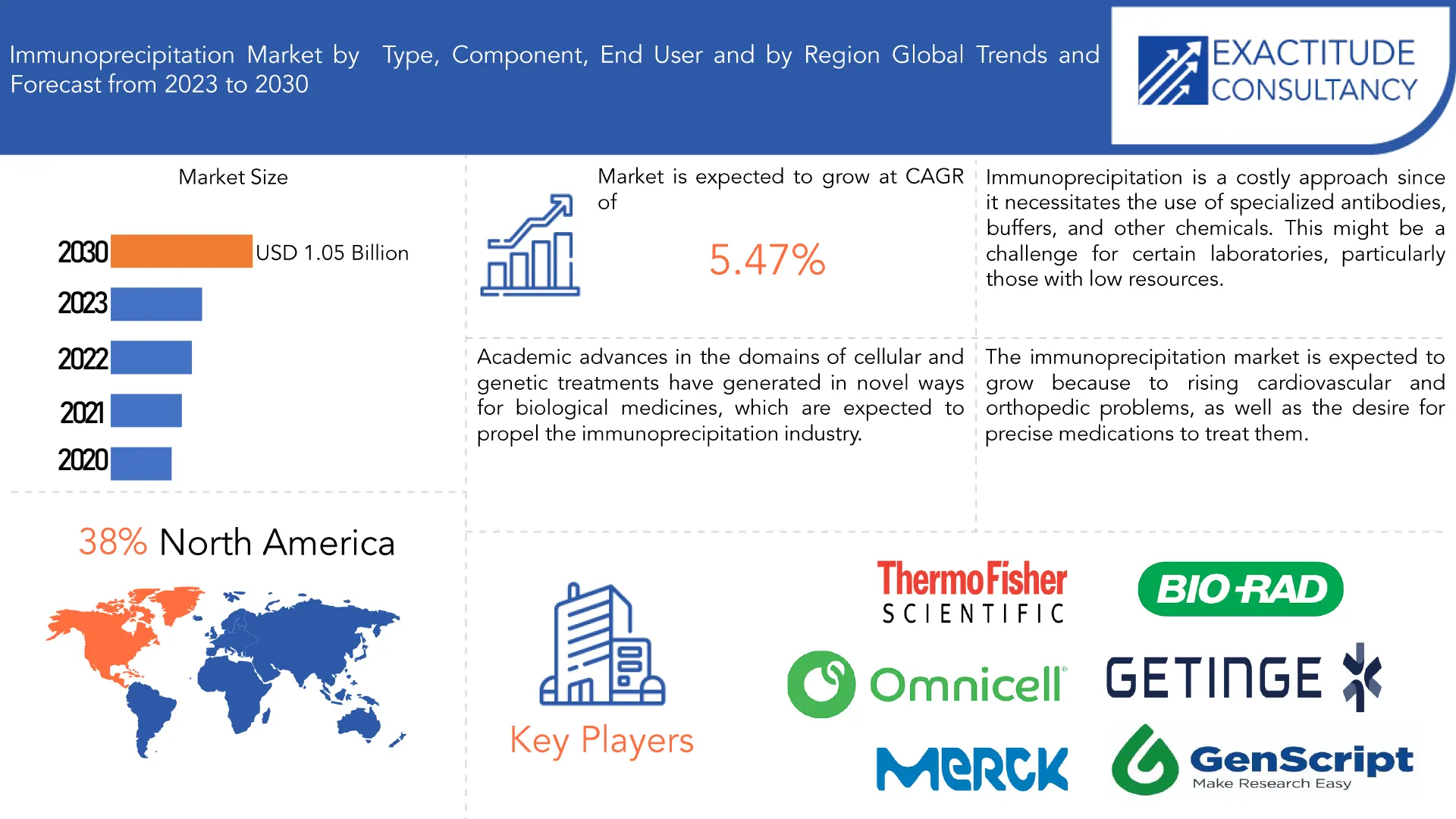
| USD 1.05 Billion by 2030, | 5.47% | North America |
| by Type | by Component | by End User | by Region |
|---|---|---|---|
|
|
|
|
SCOPE OF THE REPORT
Market Overview
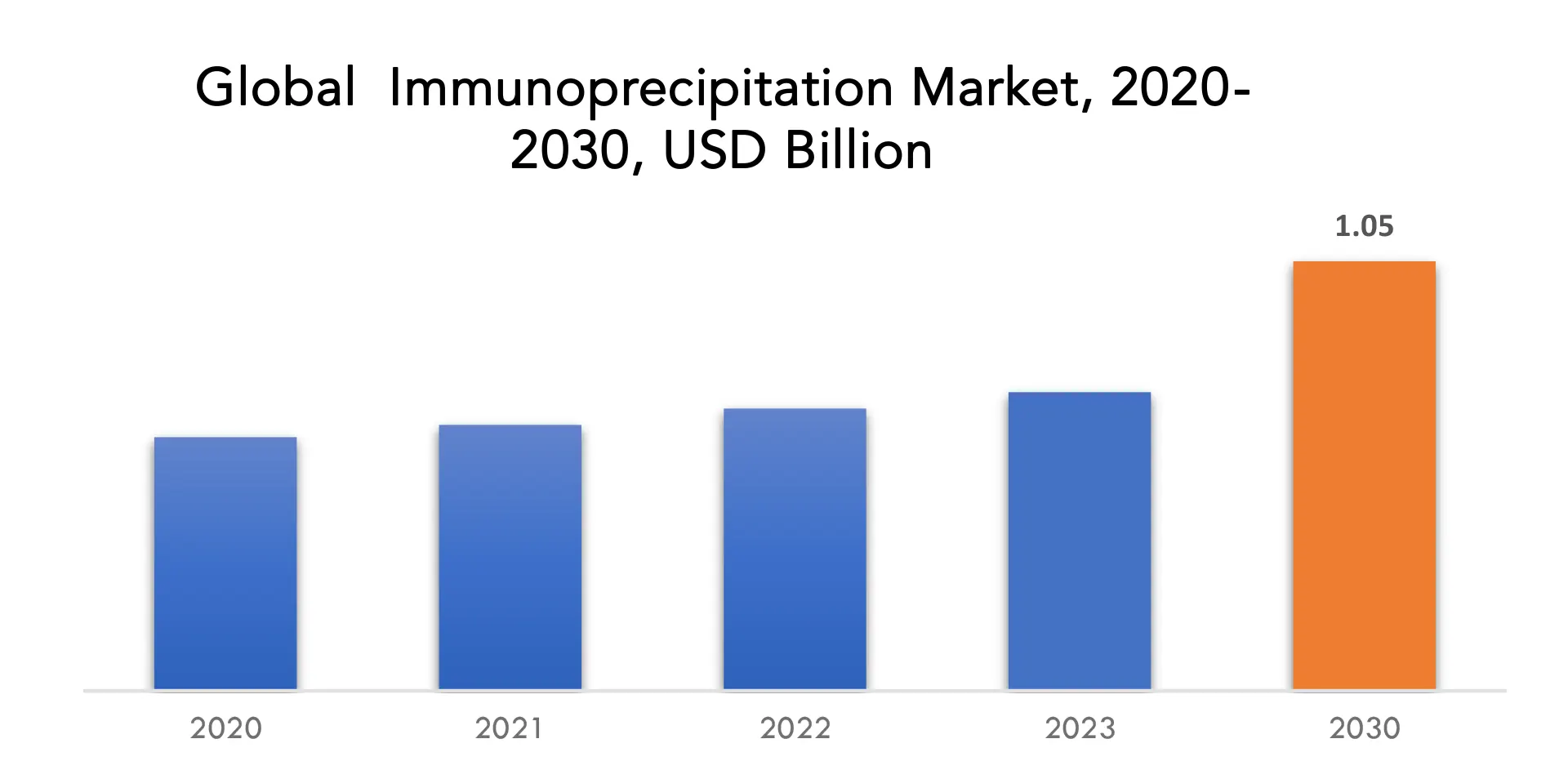
The global immunoprecipitation market is anticipated to grow from USD 0.73 Billion in 2023 to USD 1.05 Billion by 2030, at a CAGR of 5.47 % during the forecast period.
The method of collecting a protein antigen using an antibody that binds to that specific protein is known as immunoprecipitation (IP). The approach is commonly used to isolate a specific protein antigen from an assortment containing numerous other proteins. It is critical for this method that the antibody be coupled with a solid substrate that is stable. Immunoprecipitation is the process of immobilizing a particular antibody to a solid substrate, such as agarose resin or magnetic particles, for antigen affinity purification. Simply said, this method is utilized to isolate a protein (the target antigen) and assess the protein’s structure, identity, expression, and modified status. Several versions of this approach can also be used to investigate the interactions of the main antigen protein with additional proteins and nucleic acids.
The unifying objective, however, is to thoroughly investigate the interactors or biological components connected with the main antigen. Immunoprecipitation is a popular approach for separating proteins and other macromolecules from tissue or cell lysates, which are then analyzed using assay techniques such as Western Blotting. The antigen that is being targeted that has been precipitating by the antibody aids in the co-precipitation of its binding partner in co-immunoprecipitation. ChIP tests, on the other hand, are used to identify the genomic areas to which DNA-binding proteins such as histones and transcription factors are linked. RNA immunoprecipitation, like ChIP, uses cDNA and RT-PCR sequencing to identify RNA-binding proteins.
Scientific breakthroughs in genetics and biological therapies, increased expenditures in biopharmaceutical R&D activities, and expanding research initiatives in Gene expression, immunotherapy, drug development, and personalized medicine are among the other factors driving global market growth. Furthermore, the growing importance of immunoprecipitation assessment in the treatment of cancer, cardiovascular disease, and numerous central neurological illnesses is providing new opportunities for significant competitors in this market.
The rising frequency of chronic illnesses, expanding prospects for financing and investments in next-generation DNA sequencing, and increased R&D activity in genomics sector by governmental and non-governmental organizations all contribute significantly to the worldwide immunoprecipitation market growth. Immunoprecipitation methods have experienced significant technical improvements. Chromatrap Immunoprecipitation (ChIP) technology, a bead-free method of chromatin immunoprecipitation, has evolved from advances in chromatin immunoprecipitation technology. The market is expected to grow substantially as a result of epigenetic improvements enabled by ChIP-seq. Furthermore, the immunoprecipitation testing market is predicted to expand as the prevalence of autoimmune illnesses rises, necessitating antibody-antigen interaction testing. The growing prospects for government and non-government groups to fund and invest in next-generation DNA sequencing and genomics research are expected to complement immunoprecipitation demand in the future years.
During the projected period, the worldwide immunoprecipitation market will expand at a quick pace. Market expansion is being fueled by rising demand for antigen test and vaccine innovations. Furthermore, because to critical demands, most governments permitted the transport of medical instruments, test kits, and reagents during the pandemic era. This has also contributed to the ongoing selling of immunoprecipitation reagents and kits. However, because to the significant expansion in research and biological drug development, demand for immunoprecipitation is likely to rise throughout the projection period. Many biological businesses have shifted their attention to developing effective vaccines to prevent COVID-19 infections, which is projected to drive market growth.
Immunoprecipitation Market Report Scope and Segmentation
| ATTRIBUTE | DETAILS |
| Study period | 2020-2030 |
| Base year | 2022 |
| Estimated year | 2023 |
| Forecasted year | 2023-2030 |
| Historical period | 2019-2021 |
| Unit | Value (USD Billion) |
| Segmentation | By Product, By Component, By End User, and By Region |
| By Type |
|
| By Component |
|
| By End User |
|
| By Region
|
|
Immunoprecipitation Market Segmentation Analysis
The global immunoprecipitation market is divided into 4 segments type, component, end user and region. Based on type, the immunoprecipitation market is classified into RNA immunoprecipitation, co-immunoprecipitation, chromatin immunoprecipitation, and individual protein immunoprecipitation. The immunoprecipitation market is categorized into accessories, kits, and reagents based on components. By end user, the immunoprecipitation market is divided into pharma & biopharma companies, academic & research institutes, and contract research organizations.
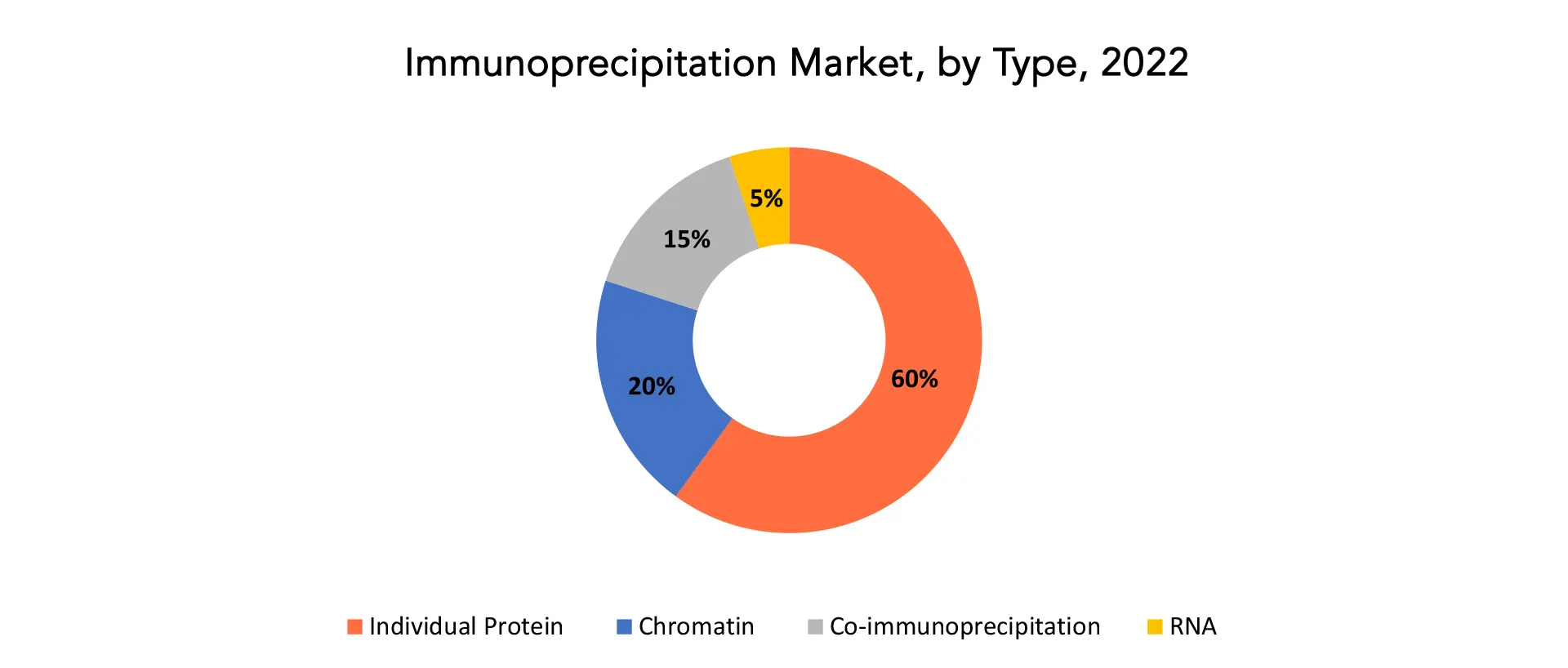
The market for immunoprecipitation in 2022 was dominated by the particular protein immunoprecipitation segment. This kind of immunoprecipitation, which removes a specific protein, is most frequently used for protein purification. The technique of separating a protein that is not understood from a complex solution containing a known protein, known as co-immunoprecipitation, is linked to protein-protein interactions. Individualized protein immunoprecipitation (IP) is a method for separating a single protein from a complicated mixture. The protein can then go through further testing, such as western blotting or mass spectrometry.
During the forecast period, the ChIP segment is estimated to account for the second largest revenue share in the worldwide Immunoprecipitation market. This is because ChIP is increasingly being used to selectively enrich certain DNA-binding proteins as well as the DNA to which those proteins are meant to bind. ChIP is used to investigate interactions throughout the entire genome or a subset of genes, as well as single protein-DNA interactions, multiple protein-DNA interactions, and combinations of these interactions. Furthermore, ChIP employs antibodies that selectively bind and detect proteins, such as histones, histone modifications, transcription variables, and cofactors, to offer information about the chromatin environment and gene transcription.
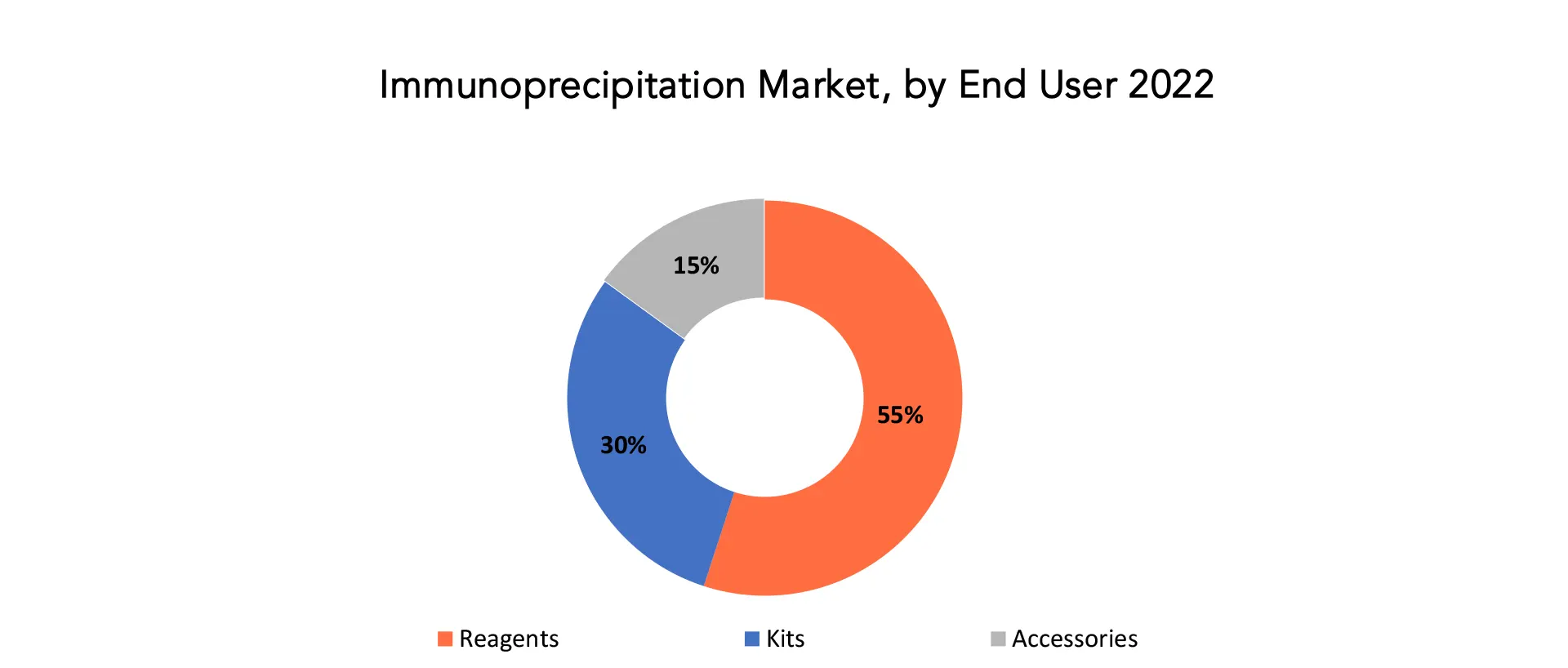
Due to the frequent usage of different antibodies, beads, and buffers, the reagents sector held the majority of the market share in 2022. Researchers frequently employ agarose beads in their efforts to purify proteins. However, the market for immunoprecipitation has been driven by a sharp increase in demand for magnetic beads since their introduction. Due to the availability of a diverse product range, the kits category is anticipated to have considerable growth throughout the projected period. Some of these include the Merck Imprint Chromatin Immunoprecipitation Kit, the Abcam Methylated DNA Immunoprecipitation (MeDIP) Kit for DNA, and the Bio-Rad Laboratories SureBeads Starter Kit. The reagents section is further divided into the following categories: buffers, magnetic beads, agarose beads, and antibodies (primary and secondary antibodies). One of the main drivers of this segment’s revenue growth is the extensive use of antibodies, buffers, and beads for various immunoprecipitation techniques, as well as the rising popularity of IP tests for the separation and purification of antigens. Another significant reason that is anticipated to drive this segment’s revenue development in the future is the rising demand for agarose and magnetic beads among research laboratories for protein purification.
Although owing to the availability of a wide range of products, the kits category is anticipated to expand at a promising CAGR over the course of the projected year. The ease, time-saving advantages, and standardized protocols of kits are what essentially drive the segmental expansion. The acceptance of kits by academic and research organizations is increasing as a consequence of their simplicity, reliability, and lack of training needs. The segmental expansion is influenced by the rising research spending in proteomics.
Immunoprecipitation Market Dynamics
Driver
One of the key factors influencing the immunoprecipitation market is the growing significance of stem cell therapy in the development of cell-based medicines.
The development of cell-based medications and the growing significance of stem cell treatment have a significant influence on the immunoprecipitation (IP) industry. By using stem cells’ regenerative potential, stem cell therapy provides a breakthrough method of treating a wide range of illnesses and injuries. Researchers largely rely on IP methods to comprehend the complex protein interactions and molecular pathways that underpin stem cell behavior in order to realize the full therapeutic potential of stem cells. IP offers vital insights into the intricate biology of stem cells by enabling researchers to extract and examine certain proteins involved in cellular differentiation, tissue regeneration, and disease modelling. likewise, strict quality control and safety evaluations are essential as stem cell treatments get closer to clinical applications. By enabling the verification of protein markers that signal the desirable properties of stem cell populations, IP plays a crucial role in these procedures, assuring the safety and efficacy of cell-based therapeutics. The need for sophisticated protein analysis techniques like IP is being driven by the increased investment in stem cell research and the expanding use of stem cell treatments, putting it as a key element of the biotechnology and regenerative medicine landscape.
Restraint
Co-immunoprecipitation disadvantages are anticipated to limit market expansion.
The drawbacks of Co-immunoprecipitation (Co-IP) are anticipated to restrain the market expansion of Immunoprecipitation (IP) techniques in general. Although co-IP is a potent tool for examining protein-protein interactions, it has a number of drawbacks that may make it difficult for other research disciplines to adopt IP techniques. First off, Co-IP is a challenging technological technique that calls for specialized knowledge and meticulous optimization. It is less accessible to people with limited knowledge or finances because to the requirement to choose highly specific antibodies, construct exact experimental settings, and painstakingly confirm results. This intrinsic complexity would deter some researchers from adopting IP techniques, which would constrain the market’s potential for development. Co-IP’s throughput and scalability have limitations. Since it is often carried out on a smaller scale, it might not be appropriate for high-throughput investigations or when working with tiny numbers of sample. The comparatively poor scalability of Co-IP may encourage researchers to look into other approaches that offer more efficiency and higher throughput when research projects call for the investigation of vast protein interaction networks. Given this situation, the difficulties with Co-IP may limit the commercial growth of IP techniques as researchers look for more scalable and accessible ways to explore protein interactions.
Opportunities
strong>Better business potential may result from integrating IP with genomics, transcriptomics, and other omics technologies.
Due of the synergistic advantages it provides to researchers from various fields, integrating immunoprecipitation (IP) with genomics, transcriptomics, and other omics technologies has enormous financial potential. A greater knowledge of biological functions, gene regulation, and protein interactions is made possible by this integration, opening up a wide range of possibilities for both technology suppliers and researchers. Researchers can map the genomic areas linked to certain protein-DNA interactions, including chromatin immunoprecipitation (ChIP), by combining IP with genomics. Critical information on gene regulation, transcription factor binding, and epigenetic changes is provided by this. Similar to how IP is used with transcriptomics, this technique enables researchers to locate and measure the RNA molecules linked to specific proteins, providing information on post-transcriptional gene regulation and RNA-protein interactions. By enabling researchers to link protein-level interactions with their downstream consequences at the genomic and transcriptome levels, such integrative techniques provide a comprehensive understanding of cellular processes and disease causes.
Immunoprecipitation Market Trends
- Automation and High-Throughput Solutions: Automation, which provides more accuracy, repeatability, and less hands-on time, is becoming more and more common in IP operations. Researchers can easily analyze enormous datasets and carry out thorough protein interaction investigations using high-throughput IP systems.
- Multiplexing for Comprehensive Analysis: To analyze several proteins or protein changes concurrently in IP studies, researchers are increasingly using multiplexing methods. This pattern simplifies research, uses fewer samples, and gives a more thorough picture of protein networks.
- Services for customization and protocol optimization are available from service providers and businesses to meet the specific requirements of researchers. To guarantee the success of IP investigations, this comprises antibody selection, protocol formulation, and assay validation.
- Integration with Mass Spectrometry: Immunoprecipitation-mass spectrometry (IP-MS), one method of integrating IP with mass spectrometry, is gaining popularity. The depth of protein interaction analysis is increased by the ability to identify interacting proteins and post-translational changes.
- Single-Cell Immunoprecipitation: As single-cell analysis gains popularity, researchers are working on methods for doing IP at the single-cell level. This development makes it possible to examine protein interactions inside individual cells, providing information on biological diversity and disease causes.
- Cross-linking and chemical intellectual property: New cross-linking agents and chemical intellectual property techniques are broadening the range of IP applications. These methods make it possible to examine momentary protein interactions and complexes that conventional IP could overlook.
Competitive Landscape
The competitive landscape in the Immunoprecipitation Market is dynamic and evolving, driven by factors such as technological advancements, healthcare infrastructure development, and the increasing demand for flexible and efficient patient care solutions. Key players in this market include established manufacturers, emerging companies, and niche players, each striving to gain a competitive edge.
- Thermo Fisher Scientific
- Merck KGaA
- Abbkine Scientific Co., Ltd.
- Abcam
- Bio-Rad Laboratories
- BioLegend
- Cell Signaling Technology.
- GenScript Biotech Corporation.
- Rockland Immunochemicals
- Geno Technology
- Omnicell, Inc.
- United Health Group
- Nexus AG
- Getinge AB
- Optum Inc.
- 3M
- Carestream Health
- MEDITECH
- GE Healthcare
- OSI Systems
Recent Developments:
27 June 2023: Thermo Fisher Scientific, the world leader in serving science, introduced the Thermo Scientific™ Metrios™ 6 Scanning Transmission Electron Microscope ((S)TEM) — a new-generation, fully automated (S)TEM metrology solution designed to help enhance productivity and deliver data quality assurance for high-volume semiconductor manufacturing.
29 March 2023: Thermo Fisher Scientific, the world leader in serving science, and Arsenal Biosciences, Inc. (ArsenalBio), a clinical-stage cell therapy company engineering advanced chimeric antigen receptor (CAR)-T cell therapies for solid tumors, today announced an update to our strategic collaboration to further the development of manufacturing processes for new cancer treatments.
Regional Analysis
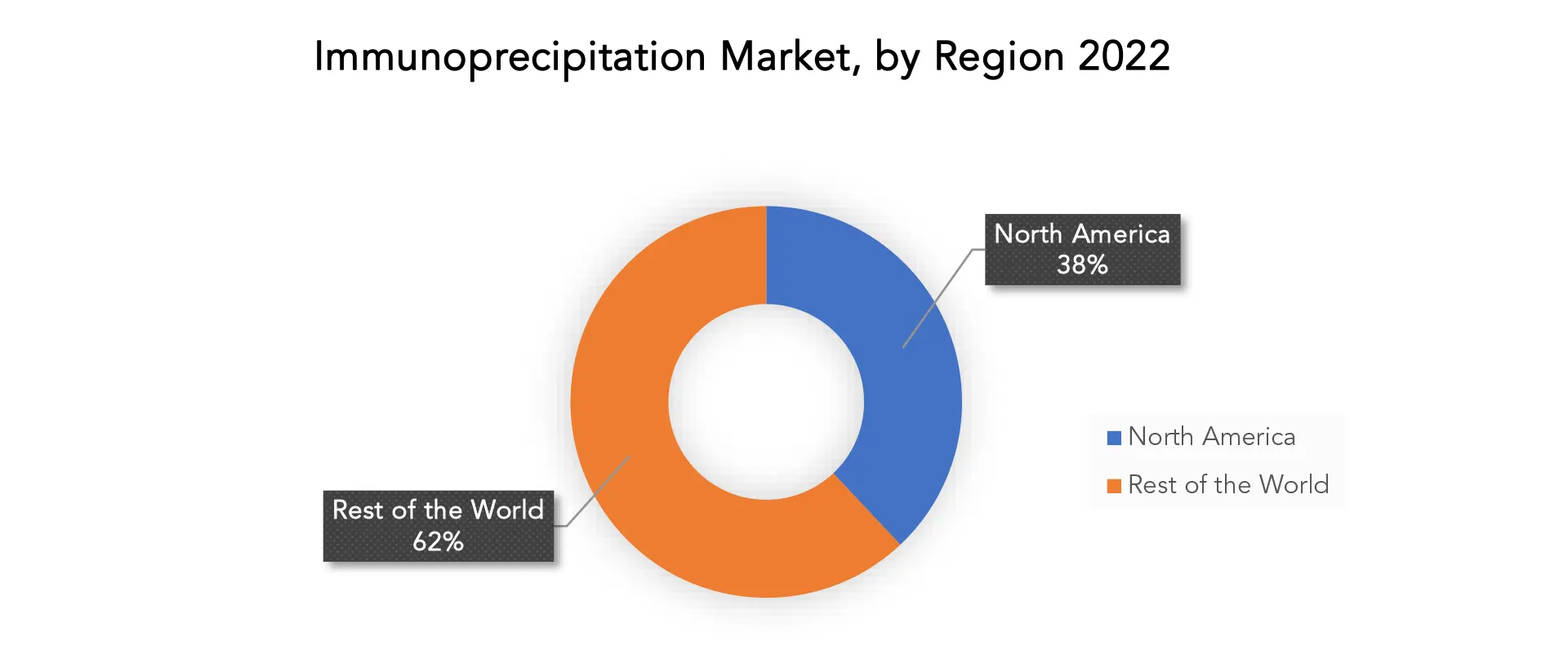
North America accounted for the largest market in the immunoprecipitation market. North America accounted for the 38 % market share of the global market value. One of the key drivers of the North American immunoprecipitation market is the presence of well-established pharmaceutical and biotechnology businesses, which fuel demand for immunoprecipitation methods in drug discovery and development. The development of the North American market is attributed to the considerable presence of university and research institutions engaged in cutting-edge proteomics and genomics research as well as the availability of major research funds and grants to promote improvements in immunoprecipitation technology. In 2022, the U.S. held the largest share of the North American market, and over the forecast period, it is anticipated to rise at a notable CAGR. Future market growth is anticipated to be supported by significant public and private sector expenditures in R&D of breakthrough genetic technologies and therapies in the United States. The growing life sciences sector promotes the broad application of immunoprecipitation techniques, which enhances the methods for protein purification. Other opportunities that entice immunoprecipitation companies to the United States include the enormous demand for DNA immunoprecipitation solutions, the presence of key market players, the quick adoption of protein complex immunoprecipitation techniques, and the expanding use of immunoprecipitation in cancer research applications.
Another significant geographical market for immunoprecipitation in the global market, APAC is anticipated to expand at the greatest CAGR over the forecast period. The need for immunoprecipitation methods in drug discovery and development in the Asia-Pacific region is driving the market expansion in APAC due to the area’s rapidly expanding biotechnology and pharmaceutical sectors. The Asia-Pacific region’s immunoprecipitation market is developing as a result of increased academic and research institution investments in proteomics and genomics R&D utilizing immunoprecipitation, a large patient population, and the rising frequency of chronic illnesses. In 2022, China accounted for the largest market share in APAC, and it is anticipated that this trend would hold during the forecast period. Infrastructure improvements made possible by technology in the healthcare sector and the use of sophisticated protein analyses.
Target Audience for Immunoprecipitation
- Hospital Administrators
- Facility Planners
- Medical Equipment Procurement Teams
- Architects
- Interior Designers
- Biomedical Engineers
- Healthcare Facility Managers
- Surgical and Operating Room Managers
- Intensive Care Unit (ICU) Directors
- Emergency Room (ER) Directors
- Anesthesiologists
- Nurses and Clinical Staff
- Healthcare Infrastructure Consultants
Segments Covered in the Immunoprecipitation Market Report
Immunoprecipitation Market by Type
- Individual Protein
- Chromatin
- Co-immunoprecipitation
- RNA
Immunoprecipitation Market by Component
- Reagents
- Kits
- Accessories
Immunoprecipitation Market by End User
- Academic & Research Institutes
- Pharma & Biopharma Companies
- Contract Research Organizations
Immunoprecipitation Market by Region
- North America
- Europe
- Asia Pacific
- South America
- Middle East and Africa
Key Question Answered
- What is the expected growth rate of the immunoprecipitation market over the next 7 years?
- Who are the major players in the immunoprecipitation market and what is their market share?
- What are the end-user industries driving market demand and what is their outlook?
- What are the opportunities for growth in emerging markets such as Asia-pacific, the middle east, and Africa?
- How is the economic environment affecting the immunoprecipitation market, including factors such as interest rates, inflation, and exchange rates?
- What is the expected impact of government policies and regulations on the immunoprecipitation market?
- What is the current and forecasted size and growth rate of the global cleanroom wipes market?
- What are the key drivers of growth in the immunoprecipitation market?
- Who are the major players in the market and what is their market share?
- What are the distribution channels and supply chain dynamics in the immunoprecipitation market?
- What are the technological advancements and innovations in the immunoprecipitation market and their impact on product development and growth?
- What are the regulatory considerations and their impact on the market?
- What are the challenges faced by players in the immunoprecipitation market and how are they addressing these challenges?
- What are the opportunities for growth and expansion in the immunoprecipitation market?
- What are the product products and specifications of leading players in the market?
Table of Content
- INTRODUCTION
- MARKET DEFINITION
- MARKET SEGMENTATION
- RESEARCH TIMELINES
- ASSUMPTIONS AND LIMITATIONS
- RESEARCH METHODOLOGY
- DATA MINING
- SECONDARY RESEARCH
- PRIMARY RESEARCH
- SUBJECT-MATTER EXPERTS’ ADVICE
- QUALITY CHECKS
- FINAL REVIEW
- DATA TRIANGULATION
- BOTTOM-UP APPROACH
- TOP-DOWN APPROACH
- RESEARCH FLOW
- DATA SOURCES
- DATA MINING
- EXECUTIVE SUMMARY
- MARKET OVERVIEW
- IMMUNOPRECIPITATION MARKET OUTLOOK
- MARKET DRIVERS
- MARKET RESTRAINTS
- MARKET OPPORTUNITIES
- IMPACT OF COVID-19 ON IMMUNOPRECIPITATION MARKET
- PORTER’S FIVE FORCES MODEL
- THREAT FROM NEW ENTRANTS
- THREAT FROM SUBSTITUTES
- BARGAINING POWER OF SUPPLIERS
- BARGAINING POWER OF CUSTOMERS
- DEGREE OF COMPETITION
- INDUSTRY VALUE CHAIN ANALYSIS
- IMMUNOPRECIPITATION MARKET OUTLOOK
- GLOBAL IMMUNOPRECIPITATION MARKET BY TYPE, 2020-2030, (USD BILLION)
- INDIVIDUAL PROTEIN
- CHROMATIN
- CO-IMMUNOPRECIPITATION
- RNA
- GLOBAL IMMUNOPRECIPITATION MARKET BY COMPONENT, 2020-2030, (USD BILLION)
- REAGENTS
- KITS
- ACCESSORIES
- GLOBAL IMMUNOPRECIPITATION MARKET BY END USER, 2020-2030, (USD BILLION)
- ACADEMIC & RESEARCH INSTITUTES
- PHARMA & BIOPHARMA COMPANIES
- CONTRACT RESEARCH ORGANIZATIONS
- GLOBAL IMMUNOPRECIPITATION MARKET BY REGION, 2020-2030, (USD BILLION)
- NORTH AMERICA
- US
- CANADA
- MEXICO
- SOUTH AMERICA
- BRAZIL
- ARGENTINA
- COLOMBIA
- REST OF SOUTH AMERICA
- EUROPE
- GERMANY
- UK
- FRANCE
- ITALY
- SPAIN
- RUSSIA
- REST OF EUROPE
- ASIA PACIFIC
- INDIA
- CHINA
- JAPAN
- SOUTH KOREA
- AUSTRALIA
- SOUTH-EAST ASIA
- REST OF ASIA PACIFIC
- MIDDLE EAST AND AFRICA
- UAE
- SAUDI ARABIA
- SOUTH AFRICA
- REST OF MIDDLE EAST AND AFRICA
- NORTH AMERICA
- COMPANY PROFILES*
(BUSINESS OVERVIEW, COMPANY SNAPSHOT, PRODUCTS OFFERED, RECENT DEVELOPMENTS)
9.1 THERMO FISHER SCIENTIFIC
9.2 MERCK KGAA
9.3 ABBKINE SCIENTIFIC CO., LTD.
9.4 ABCAM
9.5 BIO-RAD LABORATORIES
9.6 BIOLEGEND
9.7 CELL SIGNALING TECHNOLOGY.
9.8 GENSCRIPT BIOTECH CORPORATION.
9.9 ROCKLAND IMMUNOCHEMICALS
9.10 GENO TECHNOLOGY
9.11 OMNICELL, INC.
9.12 UNITED HEALTH GROUP
9.13 NEXUS AG
9.14 GETINGE AB
9.15 OPTUM INC.
9.16 3M
9.17 CARESTREAM HEALTH
9.18 MEDITECH
9.19 GE HEALTHCARE
9.20 OSI SYSTEMS
*THE COMPANY LIST IS INDICATIVE
LIST OF TABLES
TABLE 1 GLOBAL IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 2 GLOBAL IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 3 GLOBAL IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 4 GLOBAL IMMUNOPRECIPITATION MARKET BY REGIONS (USD BILLION) 2020-2030
TABLE 5 NORTH AMERICA IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 6 NORTH AMERICA IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 7 NORTH AMERICA IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 8 NORTH AMERICA IMMUNOPRECIPITATION MARKET BY COUNTRY (USD BILLION) 2020-2030
TABLE 9 US IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 10 US IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 11 US IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 12 CANADA IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 13 CANADA IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 14 CANADA IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 15 MEXICO IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 16 MEXICO IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 17 MEXICO IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 18 SOUTH AMERICA IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 19 SOUTH AMERICA IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 20 SOUTH AMERICA IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 21 SOUTH AMERICA IMMUNOPRECIPITATION MARKET BY COUNTRY (USD BILLION) 2020-2030
TABLE 22 BRAZIL IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 23 BRAZIL IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 24 BRAZIL IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 25 ARGENTINA IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 26 ARGENTINA IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 27 ARGENTINA IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 28 COLOMBIA IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 29 COLOMBIA IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 30 COLOMBIA IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 31 REST OF SOUTH AMERICA IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 32 REST OF SOUTH AMERICA IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 33 REST OF SOUTH AMERICA IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 34 ASIA-PACIFIC IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 35 ASIA-PACIFIC IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 36 ASIA-PACIFIC IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 37 ASIA-PACIFIC IMMUNOPRECIPITATION MARKET BY COUNTRY (USD BILLION) 2020-2030
TABLE 38 INDIA IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 39 INDIA IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 40 INDIA IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 41 CHINA IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 42 CHINA IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 43 CHINA IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 44 JAPAN IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 45 JAPAN IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 46 JAPAN IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 47 SOUTH KOREA IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 48 SOUTH KOREA IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 49 SOUTH KOREA IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 50 AUSTRALIA IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 51 AUSTRALIA IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 52 AUSTRALIA IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 53 SOUTH-EAST ASIA IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 54 SOUTH-EAST ASIA IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 55 SOUTH-EAST ASIA IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 56 REST OF ASIA PACIFIC IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 57 REST OF ASIA PACIFIC IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 58 REST OF ASIA PACIFIC IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 59 EUROPE IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 60 EUROPE IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 61 EUROPE IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 62 EUROPE IMMUNOPRECIPITATION MARKET BY COUNTRY (USD BILLION) 2020-2030
TABLE 63 GERMANY IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 64 GERMANY IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 65 GERMANY IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 66 UK IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 67 UK IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 68 UK IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 69 FRANCE IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 70 FRANCE IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 71 FRANCE IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 72 ITALY IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 73 ITALY IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 74 ITALY IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 75 SPAIN IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 76 SPAIN IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 77 SPAIN IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 78 RUSSIA IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 79 RUSSIA IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 80 RUSSIA IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 81 REST OF EUROPE IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 82 REST OF EUROPE IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 83 REST OF EUROPE IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 84 MIDDLE EAST AND AFRICA IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 85 MIDDLE EAST AND AFRICA IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 86 MIDDLE EAST AND AFRICA IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 87 MIDDLE EAST AND AFRICA IMMUNOPRECIPITATION MARKET BY COUNTRY (USD BILLION) 2020-2030
TABLE 88 UAE IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 89 UAE IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 90 UAE IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 91 SAUDI ARABIA IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 92 SAUDI ARABIA IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 93 SAUDI ARABIA IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 94 SOUTH AFRICA IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 95 SOUTH AFRICA IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 96 SOUTH AFRICA IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
TABLE 97 REST OF MIDDLE EAST AND AFRICA IMMUNOPRECIPITATION MARKET BY TYPE (USD BILLION) 2020-2030
TABLE 98 REST OF MIDDLE EAST AND AFRICA IMMUNOPRECIPITATION MARKET BY COMPONENT (USD BILLION) 2020-2030
TABLE 99 REST OF MIDDLE EAST AND AFRICA IMMUNOPRECIPITATION MARKET BY END USER (USD BILLION) 2020-2030
LIST OF FIGURES
FIGURE 1 MARKET DYNAMICS
FIGURE 2 MARKET SEGMENTATION
FIGURE 3 REPORT TIMELINES: YEARS CONSIDERED
FIGURE 4 DATA TRIANGULATION
FIGURE 5 BOTTOM-UP APPROACH
FIGURE 6 TOP-DOWN APPROACH
FIGURE 7 RESEARCH FLOW
FIGURE 8 GLOBAL IMMUNOPRECIPITATION MARKET BY TYPE USD BILLION, 2020-2030
FIGURE 9 GLOBAL IMMUNOPRECIPITATION MARKET BY COMPONENT USD BILLION, 2020-2030
FIGURE 10 GLOBAL IMMUNOPRECIPITATION MARKET BY END USER USD BILLION, 2020-2030
FIGURE 11 GLOBAL IMMUNOPRECIPITATION MARKET BY REGION USD BILLION, 2020-2030
FIGURE 12 PORTER’S FIVE FORCES MODEL
FIGURE 13 GLOBAL IMMUNOPRECIPITATION MARKET BY TYPE USD BILLION, 2022
FIGURE 14 GLOBAL IMMUNOPRECIPITATION MARKET BY COMPONENT USD BILLION, 2022
FIGURE 15 GLOBAL IMMUNOPRECIPITATION MARKET BY END USER USD BILLION, 2022
FIGURE 16 GLOBAL IMMUNOPRECIPITATION MARKET BY REGION USD BILLION, 2022
FIGURE 17 MARKET SHARE ANALYSIS
FIGURE 18 THERMO FISHER SCIENTIFIC: COMPANY SNAPSHOT
FIGURE 19 MERCK KGAA: COMPANY SNAPSHOT
FIGURE 20 ABBKINE SCIENTIFIC CO., LTD.: COMPANY SNAPSHOT
FIGURE 21 ABCAM: COMPANY SNAPSHOT
FIGURE 22 BIO-RAD LABORATORIES: COMPANY SNAPSHOT
FIGURE 23 BIOLEGEND: COMPANY SNAPSHOT
FIGURE 24 CELL SIGNALING TECHNOLOGY.: COMPANY SNAPSHOT
FIGURE 25 GENSCRIPT BIOTECH CORPORATION.: COMPANY SNAPSHOT
FIGURE 26 ROCKLAND IMMUNOCHEMICALS: COMPANY SNAPSHOT
FIGURE 27 GENO TECHNOLOGY: COMPANY SNAPSHOT
FIGURE 28 OMNICELL, INC.: COMPANY SNAPSHOT
FIGURE 29 UNITED HEALTH GROUP: COMPANY SNAPSHOT
FIGURE 30 NEXUS AG: COMPANY SNAPSHOT
FIGURE 31 GETINGE AB: COMPANY SNAPSHOT
FIGURE 32 OPTUM INC.: COMPANY SNAPSHOT
FIGURE 33 3M: COMPANY SNAPSHOT
FIGURE 34 CARESTREAM HEALTH: COMPANY SNAPSHOT
FIGURE 35 MEDITECH: COMPANY SNAPSHOT
FIGURE 36 GE HEALTHCARE: COMPANY SNAPSHOT
FIGURE 37 OSI SYSTEMS: COMPANY SNAPSHOT
FAQ
The global immunoprecipitation market is anticipated to grow from USD 0.73 Billion in 2023 to USD 1.05 Billion by 2030, at a CAGR of 5.47 % during the forecast period.
North America accounted for the largest market in the immunoprecipitation market. North America accounted for 38 % market share of the global market value.
Thermo Fisher Scientific, Merck KGaA, Abbkine Scientific Co., Ltd., Abcam, Bio-Rad Laboratories, BioLegend, Cell Signaling Technology., GenScript Biotech Corporation., Rockland Immunochemicals, Geno Technology, Omnicell, Inc., United Health Group, Nexus AG, Getinge AB, Optum Inc., 3M, Carestream Health, MEDITECH, GE Healthcare, OSI Systems.
Automation, which provides more accuracy, repeatability, and less hands-on time, is becoming more and more common in IP operations. Researchers can easily analyze enormous datasets and carry out thorough protein interaction investigations using high-throughput IP systems.
In-Depth Database
Our Report’s database covers almost all topics of all regions over the Globe.
Recognised Publishing Sources
Tie ups with top publishers around the globe.
Customer Support
Complete pre and post sales
support.
Safe & Secure
Complete secure payment
process.
